|
Is a grain of salt a single molecule, or is it just a bunch which happens to stick together? If you've been following us, you'd know that a molecule is a group of atoms which had bonded together by their respective covalent electrons. It then begs the question: how big can molecules get?
The answer? As big as you want... |
"There are three things extremely hard: steel, a diamond, and to know one's self."
Benjamin Franklin, Poor Richard’s Almanack (1750)
Covalent solids are formed by networks or chains of atoms or molecules held together by covalent bonds. A perfect single crystal of a covalent solid is therefore a single giant molecule. For example, the structure of diamonds, consists of hybridized carbon atoms, each bonded to four other carbon atoms in a tetrahedral array to create a giant network. The carbon atoms form six-membered rings.
The unit cell of diamond can be described as an fcc array of carbon atoms with four additional carbon atoms inserted into four of the tetrahedral holes. It thus has a zinc blende structure, except that in zinc blende the atoms that compose the array are sulfur and the atoms in the tetrahedral holes are zinc. Elemental silicon has the same structure, as does silicon carbide (SiC), which has alternating C and Si atoms. The structure of crystalline quartz (SiO2), shown in Section 12.1, can be viewed as being derived from the structure of silicon by inserting an oxygen atom between each pair of silicon atoms.
All compounds with the diamond and related structures are hard, high-melting-point solids that are not easily deformed. Instead, they tend to shatter when subjected to large stresses, and they usually do not conduct electricity very well. In fact, diamond (melting point = 3500°C at 63.5 atm) is one of the hardest substances known, and silicon carbide (melting point = 2986°C) is used commercially as an abrasive in sandpaper and grinding wheels. It is difficult to deform or melt these and related compounds because strong covalent (C–C or Si–Si) or polar covalent (Si–C or Si–O) bonds must be broken, which requires a large input of energy.
To completely describe the bonding in graphite, we need a molecular orbital approach similar to the one used for benzene. In fact, the C–C distance in graphite is slightly longer than the distance in benzene (139.5 pm), consistent with a net carbon–carbon bond order of 1.33. In graphite, the two-dimensional planes of carbon atoms are stacked to form a three-dimensional solid; only London dispersion forces hold the layers together. As a result, graphite exhibits properties typical of both covalent and molecular solids. Due to strong covalent bonding within the layers, graphite has a very high melting point, as expected for a covalent solid (it actually sublimes at about 3915°C). It is also very soft; the layers can easily slide past one another because of the weak interlayer interactions. Consequently, graphite is used as a lubricant and as the “lead” in pencils; the friction between graphite and a piece of paper is sufficient to leave a thin layer of carbon on the paper. Graphite is unusual among covalent solids in that its electrical conductivity is very high parallel to the planes of carbon atoms because of delocalized C–C π bonding. Finally, graphite is black because it contains an immense number of alternating double bonds, which results in a very small energy difference between the individual molecular orbitals. Thus light of virtually all wavelengths is absorbed. Diamond, on the other hand, is colorless when pure because it has no delocalized electrons.
Carbon: An example of an Covalent Network Solid
In network solids, conventional chemical bonds hold the chemical subunits together. The bonding between chemical subunits, however, is identical to that within the subunits, resulting in a continuous network of chemical bonds. One common examples of network solids are diamond (a form of pure carbon) Carbon exists as a pure element at room temperature in three different forms: graphite (the most stable form), diamond, and fullerene.
The unit cell of diamond can be described as an fcc array of carbon atoms with four additional carbon atoms inserted into four of the tetrahedral holes. It thus has a zinc blende structure, except that in zinc blende the atoms that compose the array are sulfur and the atoms in the tetrahedral holes are zinc. Elemental silicon has the same structure, as does silicon carbide (SiC), which has alternating C and Si atoms. The structure of crystalline quartz (SiO2), shown in Section 12.1, can be viewed as being derived from the structure of silicon by inserting an oxygen atom between each pair of silicon atoms.
All compounds with the diamond and related structures are hard, high-melting-point solids that are not easily deformed. Instead, they tend to shatter when subjected to large stresses, and they usually do not conduct electricity very well. In fact, diamond (melting point = 3500°C at 63.5 atm) is one of the hardest substances known, and silicon carbide (melting point = 2986°C) is used commercially as an abrasive in sandpaper and grinding wheels. It is difficult to deform or melt these and related compounds because strong covalent (C–C or Si–Si) or polar covalent (Si–C or Si–O) bonds must be broken, which requires a large input of energy.
To completely describe the bonding in graphite, we need a molecular orbital approach similar to the one used for benzene. In fact, the C–C distance in graphite is slightly longer than the distance in benzene (139.5 pm), consistent with a net carbon–carbon bond order of 1.33. In graphite, the two-dimensional planes of carbon atoms are stacked to form a three-dimensional solid; only London dispersion forces hold the layers together. As a result, graphite exhibits properties typical of both covalent and molecular solids. Due to strong covalent bonding within the layers, graphite has a very high melting point, as expected for a covalent solid (it actually sublimes at about 3915°C). It is also very soft; the layers can easily slide past one another because of the weak interlayer interactions. Consequently, graphite is used as a lubricant and as the “lead” in pencils; the friction between graphite and a piece of paper is sufficient to leave a thin layer of carbon on the paper. Graphite is unusual among covalent solids in that its electrical conductivity is very high parallel to the planes of carbon atoms because of delocalized C–C π bonding. Finally, graphite is black because it contains an immense number of alternating double bonds, which results in a very small energy difference between the individual molecular orbitals. Thus light of virtually all wavelengths is absorbed. Diamond, on the other hand, is colorless when pure because it has no delocalized electrons.
Carbon: An example of an Covalent Network Solid
In network solids, conventional chemical bonds hold the chemical subunits together. The bonding between chemical subunits, however, is identical to that within the subunits, resulting in a continuous network of chemical bonds. One common examples of network solids are diamond (a form of pure carbon) Carbon exists as a pure element at room temperature in three different forms: graphite (the most stable form), diamond, and fullerene.
|
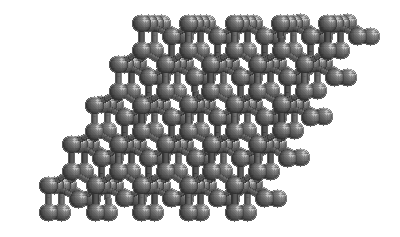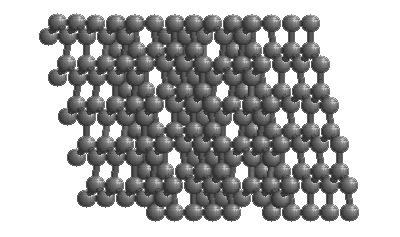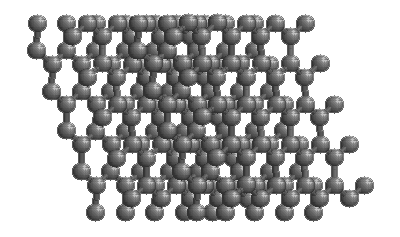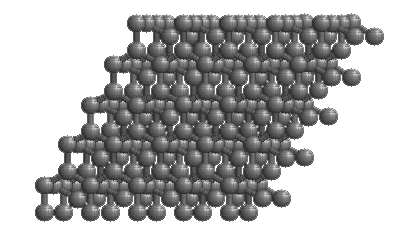
Diamonds
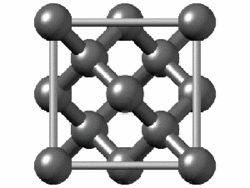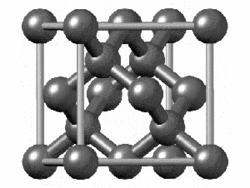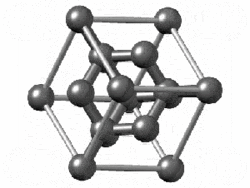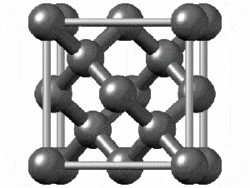
The structure of diamond is shown at the right in a "ball-and-stick" format. The balls represent the carbon atoms and the sticks represent a covalent bond. Be aware that in the "ball-and-stick" representation the size of the balls do not accurately represent the size of carbon atoms. In addition, a single stick is drawn to represent a covalent bond irrespective of whether the bond is a single, double, or triple bond or requires resonance structures to represent. In the diamond structure, all bonds are single covalent bonds ( σσ bonds). The "space-filling" format is an alternate representation that displays atoms as spheres with a radius equal to the van der Waals radius, thus providing a better sense of the size of the atoms. Notice that diamond is a network solid. The entire solid is an "endless" repetition of carbon atoms bonded to each other by covalent bonds. |
|
Graphite
Graphite has a layered, planar structure. The individual layers are called graphene. In each layer, the carbon atoms are arranged in a honeycomb lattice with separation of 0.142 nm, and the distance between planes is 0.335 nm. Atoms in the plane are bonded covalently, with only three of the four potential bonding sites satisfied. The fourth electron is free to migrate in the plane, making graphite electrically conductive. However, it does not conduct in a direction at right angles to the plane. Bonding between layers is via weak van der Waals bonds, which allows layers of graphite to be easily separated, or to slide past each other. |
|
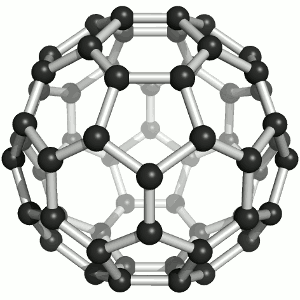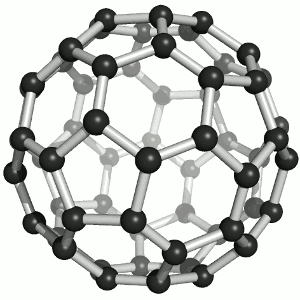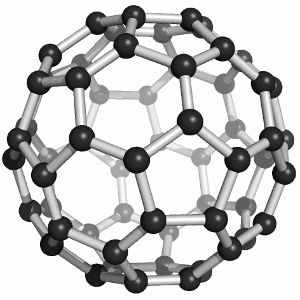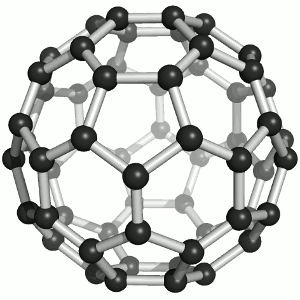
Fullerenes
Until the mid 1980's, pure carbon was thought to exist in two forms: graphite and diamond. The discovery of C60 molecules in interstellar dust in 1985 added a third form to this list. The existence of C60, which resembles a football, had been hypothesized by theoretians for many years. In the late 1980's synthetic methods were developed for the synthesis of C60, and the ready availability of this form of carbon led to extensive research into its properties. The C60 molecule, is called buckminsterfullerene, though the shorter name fullerene is often used. The name is a tribute to the American architect R. Buckminster Fuller, who is famous for designing and constructing geodesic domes which bear a close similarity to the structure of C60. As is evident from the display, C60 is a sphere composed of six-member and five-member carbon rings. These balls are sometimes fondly referred to as "bucky balls". It should be noted that fullerenes are an entire class of pure carbon compounds rather than a single compound. A distorted sphere containing more than 60 carbon atoms have also been found, and it is also possible to create long tubes. All of these substances are pure carbon. |
Ponder this
How do the properties of a network solid change, if any, as they increase in size?
Would a simple change in structure allow for similar changes in properties for any element and/or compound?
Discuss
Get a table of elements and rank them by their respective number of valence electrons. Discuss the possibility of these elements producing network solid by themselves or as compounds. Compare these hypothetical network solids in terms of their properties (e.g. hardness, melting point, chemical reactivity). Contrast these hypothetical properties with those of their elemental forms, and posit possible applications of these new network solids.
Further readings
"Each blade a single crystal", an article from American Scientist on the use of single crystals in turbine engines.
"Can a single molecule have a state?", a question posed on StackExchange's physics board which resulted in a few very interesting hypotheses.