|
Nuclear chemistry is a field that straddles both physics and chemistry. This holds no surprise as chemistry is, in a way, a subset of applied physics. Therefore things that are usually linked to physics (radiation, and subatomic particles) can and will affect things we interact with in our lives - thing that are ultimately chemicals.
Just don't press your luck on becoming the Incredible Hulk. |
"As chemists, we must rename [our] scheme and insert the symbols Ba, La, Ce in place of Ra, Ac, Th. As nuclear chemists closely associated with physics, we cannot yet convince ourselves to make this leap, which contradicts all previous experience in nuclear physics."
Otto Hahn (1879 - 1968)
Most of us have at least one device in our homes that guards our safety and, at the same time, depends on radioactivity to operate properly. This device is a smoke detector.
A typical smoke detector contains an electric circuit that includes two metal plates about 1 cm apart. A battery in the circuit creates a voltage between the plates. Next to the plates is a small disk containing a tiny amount (∼0.0002 g) of the radioactive element americium. The radioactivity of americium ionizes the air between the plates, causing a tiny current to constantly flow between them. (This constant drain on the battery explains why the batteries in smoke detectors should be replaced regularly, whether the alarm has been triggered or not.)
When particles of smoke from a fire enter the smoke detector, they interfere with the ions between the metal plates, interrupting the flow of current. When the current drops beneath a set value, another circuit triggers a loud alarm, warning of the possible presence of fire.
Although radioactive, the americium in a smoke detector is embedded in plastic and is not harmful unless the plastic package is taken apart, which is unlikely. Although many people have an unfounded fear of radioactivity, smoke detectors save thousands of lives every year.
Radioactive isotopes have a variety of applications. Generally, however, they are useful because either we can detect their radioactivity or we can use the energy they release.
Radioactive isotopes are effective tracers because their radioactivity is easy to detect. A tracer is a substance that can be used to follow the pathway of that substance through some structure. For instance, leaks in underground water pipes can be discovered by running some tritium-containing water through the pipes and then using a Geiger counter to locate any radioactive tritium subsequently present in the ground around the pipes. (Recall that tritium is a radioactive isotope of hydrogen.)
Tracers can also be used to follow the steps of a complex chemical reaction. After incorporating radioactive atoms into reactant molecules, scientists can track where the atoms go by following their radioactivity. One excellent example of this is the use of carbon-14 to determine the steps involved in photosynthesis in plants. We know these steps because researchers followed the progress of carbon-14 throughout the process.
A typical smoke detector contains an electric circuit that includes two metal plates about 1 cm apart. A battery in the circuit creates a voltage between the plates. Next to the plates is a small disk containing a tiny amount (∼0.0002 g) of the radioactive element americium. The radioactivity of americium ionizes the air between the plates, causing a tiny current to constantly flow between them. (This constant drain on the battery explains why the batteries in smoke detectors should be replaced regularly, whether the alarm has been triggered or not.)
When particles of smoke from a fire enter the smoke detector, they interfere with the ions between the metal plates, interrupting the flow of current. When the current drops beneath a set value, another circuit triggers a loud alarm, warning of the possible presence of fire.
Although radioactive, the americium in a smoke detector is embedded in plastic and is not harmful unless the plastic package is taken apart, which is unlikely. Although many people have an unfounded fear of radioactivity, smoke detectors save thousands of lives every year.
Radioactive isotopes have a variety of applications. Generally, however, they are useful because either we can detect their radioactivity or we can use the energy they release.
Radioactive isotopes are effective tracers because their radioactivity is easy to detect. A tracer is a substance that can be used to follow the pathway of that substance through some structure. For instance, leaks in underground water pipes can be discovered by running some tritium-containing water through the pipes and then using a Geiger counter to locate any radioactive tritium subsequently present in the ground around the pipes. (Recall that tritium is a radioactive isotope of hydrogen.)
Tracers can also be used to follow the steps of a complex chemical reaction. After incorporating radioactive atoms into reactant molecules, scientists can track where the atoms go by following their radioactivity. One excellent example of this is the use of carbon-14 to determine the steps involved in photosynthesis in plants. We know these steps because researchers followed the progress of carbon-14 throughout the process.
Radioactive Dating
Radioactive isotopes are useful for establishing the ages of various objects. The half-life of radioactive isotopes is unaffected by any environmental factors, so the isotope acts like an internal clock. For example, if a rock is analyzed and is found to contain a certain amount of uranium-235 and a certain amount of its daughter isotope, we can conclude that a certain fraction of the original uranium-235 has radioactively decayed. If half of the uranium has decayed, then the rock has an age of one half-life of uranium-235, or about 4.5 × 109 y. Many analyses like this, using a wide variety of isotopes, have indicated that age of the earth itself is over 4 × 109 y. In another interesting example of radioactive dating, hydrogen-3 dating has been used to verify the stated vintages of some old fine wines.
One isotope, carbon-14, is particularly useful in determining the age of once-living artifacts. A tiny amount of carbon-14 is produced naturally in the upper reaches of the atmosphere, and living things incorporate some of it into their tissues, building up to a constant, albeit very low, level. Once a living thing dies, it no longer acquires carbon-14; as time passes the carbon-14 that was in the tissues decays. (The half-life of carbon-14 is 5,370 y.) If a once-living artifact is discovered and analyzed many years after its death and the remaining carbon-14 is compared to the known constant level, an approximate age of the artifact can be determined. Using such methods, scientists were able to use radiocarbon dating to show that the age of a mummified body found in the ice of the Alps was 5,300 y.
Radioactive isotopes are useful for establishing the ages of various objects. The half-life of radioactive isotopes is unaffected by any environmental factors, so the isotope acts like an internal clock. For example, if a rock is analyzed and is found to contain a certain amount of uranium-235 and a certain amount of its daughter isotope, we can conclude that a certain fraction of the original uranium-235 has radioactively decayed. If half of the uranium has decayed, then the rock has an age of one half-life of uranium-235, or about 4.5 × 109 y. Many analyses like this, using a wide variety of isotopes, have indicated that age of the earth itself is over 4 × 109 y. In another interesting example of radioactive dating, hydrogen-3 dating has been used to verify the stated vintages of some old fine wines.
One isotope, carbon-14, is particularly useful in determining the age of once-living artifacts. A tiny amount of carbon-14 is produced naturally in the upper reaches of the atmosphere, and living things incorporate some of it into their tissues, building up to a constant, albeit very low, level. Once a living thing dies, it no longer acquires carbon-14; as time passes the carbon-14 that was in the tissues decays. (The half-life of carbon-14 is 5,370 y.) If a once-living artifact is discovered and analyzed many years after its death and the remaining carbon-14 is compared to the known constant level, an approximate age of the artifact can be determined. Using such methods, scientists were able to use radiocarbon dating to show that the age of a mummified body found in the ice of the Alps was 5,300 y.
|
Irradiation of Food
The radiation emitted by some radioactive substances can be used to kill microorganisms on a variety of foodstuffs, extending the shelf life of these products. Produce such as tomatoes, mushrooms, sprouts, and berries are irradiated with the emissions from cobalt-60 or cesium-137. This exposure kills a lot of the bacteria that cause spoilage, so the produce stays fresh longer. Eggs and some meat, such as beef, pork, and poultry, can also be irradiated. Contrary to the belief of some people, irradiation of food does not make the food itself radioactive. |
|
Medical Applications
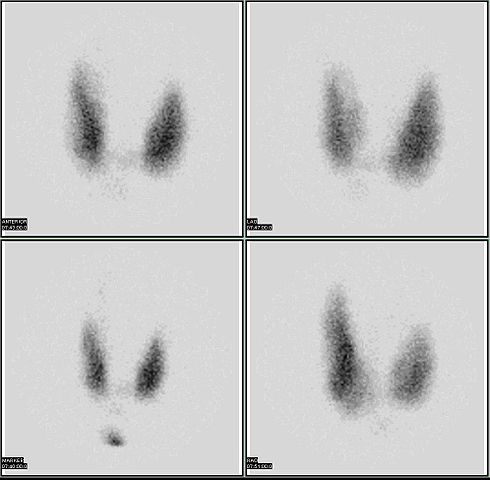
Radioactive isotopes have numerous medical applications—diagnosing and treating illness and diseases. One example of a diagnostic application is using radioactive iodine-131 to test for thyroid activity. The thyroid gland in the neck is one of the few places in the body with a significant concentration of iodine. To evaluate thyroid activity, a measured dose of 131I is administered to a patient, and the next day a scanner is used to measure the amount of radioactivity in the thyroid gland. The amount of radioactive iodine that collects there is directly related to the activity of the thyroid, allowing trained physicians to diagnose both hyperthyroidism and hypothyroidism. Iodine-131 has a half-life of only 8 d, so the potential for damage due to exposure is minimal. Technetium-99 can also be used to test thyroid function. Bones, the heart, the brain, the liver, the lungs, and many other organs can be imaged in similar ways by using the appropriate radioactive isotope. |
Nuclear Energy
Nuclear changes occur with a simultaneous release of energy. Where does this energy come from? If we could precisely measure the masses of the reactants and products of a nuclear reaction, we would notice that the amount of mass drops slightly in the conversion from reactants to products.
A nuclear reactorAn apparatus designed to carefully control the progress of a nuclear reaction and extract the resulting energy for useful purposes. is an apparatus designed to carefully control the progress of a nuclear reaction and extract the resulting energy for useful purposes. The energy from the controlled nuclear reaction converts water into high-pressure steam, which is used to run turbines that generate electricity.
The two main components of the power plant are the nuclear reactor itself and the steam-driven turbine and electricity generator.
Although the fission of large nuclei can produce different products, on average the fission of uranium produces two more free neutrons than were present to begin with. These neutrons can themselves stimulate other uranium nuclei to undergo fission, releasing yet more energy and even more neutrons, which can in turn induce even more uranium fission. A single neutron can thus begin a process that grows exponentially in a phenomenon called a chain reactionAn exponential growth in a phenomenon.
Because energy is produced with each fission event, energy is also produced exponentially and in an uncontrolled fashion. The quick production of energy creates an explosion. This is the mechanism behind the atomic bombA weapon that depends on a nuclear chain reaction to generate immense forces.. (The first controlled chain reaction was achieved on December 2, 1942, in an experiment supervised by Enrico Fermi in a laboratory underneath the football stadium at the University of Chicago.)
Although fairly simple in theory, an atomic bomb is difficult to produce, in part because uranium-235, the isotope that undergoes fission, makes up only 0.7% of natural uranium; the rest is mostly uranium-238, which does not undergo fission. (Remember that the radioactive process that a nucleus undergoes is characteristic of the isotope.) To make uranium useful for nuclear reactors, the uranium in uranium-235 must be enriched to about 3%. The enrichment of uranium is a laborious and costly series of physical and chemical separations. To be useful in an atomic bomb, uranium must be enriched to 70% or more. At lesser concentrations, the chain reaction cannot sustain itself, so no explosion is produced.
Nuclear changes occur with a simultaneous release of energy. Where does this energy come from? If we could precisely measure the masses of the reactants and products of a nuclear reaction, we would notice that the amount of mass drops slightly in the conversion from reactants to products.
A nuclear reactorAn apparatus designed to carefully control the progress of a nuclear reaction and extract the resulting energy for useful purposes. is an apparatus designed to carefully control the progress of a nuclear reaction and extract the resulting energy for useful purposes. The energy from the controlled nuclear reaction converts water into high-pressure steam, which is used to run turbines that generate electricity.
The two main components of the power plant are the nuclear reactor itself and the steam-driven turbine and electricity generator.
Although the fission of large nuclei can produce different products, on average the fission of uranium produces two more free neutrons than were present to begin with. These neutrons can themselves stimulate other uranium nuclei to undergo fission, releasing yet more energy and even more neutrons, which can in turn induce even more uranium fission. A single neutron can thus begin a process that grows exponentially in a phenomenon called a chain reactionAn exponential growth in a phenomenon.
Because energy is produced with each fission event, energy is also produced exponentially and in an uncontrolled fashion. The quick production of energy creates an explosion. This is the mechanism behind the atomic bombA weapon that depends on a nuclear chain reaction to generate immense forces.. (The first controlled chain reaction was achieved on December 2, 1942, in an experiment supervised by Enrico Fermi in a laboratory underneath the football stadium at the University of Chicago.)
Although fairly simple in theory, an atomic bomb is difficult to produce, in part because uranium-235, the isotope that undergoes fission, makes up only 0.7% of natural uranium; the rest is mostly uranium-238, which does not undergo fission. (Remember that the radioactive process that a nucleus undergoes is characteristic of the isotope.) To make uranium useful for nuclear reactors, the uranium in uranium-235 must be enriched to about 3%. The enrichment of uranium is a laborious and costly series of physical and chemical separations. To be useful in an atomic bomb, uranium must be enriched to 70% or more. At lesser concentrations, the chain reaction cannot sustain itself, so no explosion is produced.
Fusion is a nuclear process in which small nuclei are combined into larger nuclei, releasing energy. is another nuclear process that can be used to produce energy. In this process, smaller nuclei are combined to make larger nuclei, with an accompanying release of energy. One example is hydrogen fusion, which makes helium.
The amount of energy given off per mole of reactant is only one-tenth of the amount given off by the fission of 1 mol of uranium-235. On a mass (per gram) basis, however, hydrogen fusion gives off 10 times more energy than fission does. In addition, the product of fission is helium gas, not a wide range of isotopes (some of which are also radioactive) produced by fission.
Fusion occurs in nature: The sun and other stars use fusion as their ultimate energy source. Fusion is also the basis of very destructive weapons that have been developed by several countries around the world. However, one current goal is to develop a source of controlled fusion for use as an energy source. The practical problem is that to perform fusion, extremely high pressures and temperatures are necessary. Currently, the only known stable systems undergoing fusion are the interiors of stars. The conditions necessary for fusion can be created using an atomic bomb, but the resulting fusion is uncontrollable (and the basis for another type of bomb, a hydrogen bomb). Currently, researchers are looking for safe, controlled ways for producing useful energy using fusion.
Ponder this
If physics can affect chemistry, can chemistry affect physics?
The medical applications of nuclear chemistry seems quite distant from the source of these discoveries (physics and biology aren't directly relatable). How did the medical field discover the link?
Discuss
There have been some public resistance on the irradiation of food. Discuss with the class on what it is and how it is done. What are the public concerns surrounding this practice and are they founded? Discuss on similar cases of public apprehension on other application of nuclear chemistry.